TA Scan’s intuitive interface has been designed to align with user workflow. This allows your team to quickly access the data that is most relevant to your project. Next, you can easily analyze this data within the system to drive the actionable insights you require.

Clinical Feasibility & Site Identification
Competitive Intelligence & Disease Landscaping
KOL Identification & Profiling
Single Source of Truth
With TA Scan’s AI and ML power, teams working on clinical, medical affairs, marketing and commercial projects never have to comb through data silos and rely on anecdotal evidence ever again. TA Scan is an intuitive, all-in-one tool that collects, aggregates, and analyzes a plethora of private and public data sources in one comprehensive place. Mine data from hundreds of public domain sources (e.g. clinicaltrials.gov, PubMed, Sunshine Act, Medicare, etc.), including 450,000 unique clinical trials taking place globally.

API for Flexible Data Delivery
Anju understands your need for flexibility in accessing data. You can access TA scan data through its intuitive user interface, or via an API as an additional data service in broader data lakes of public and private data.
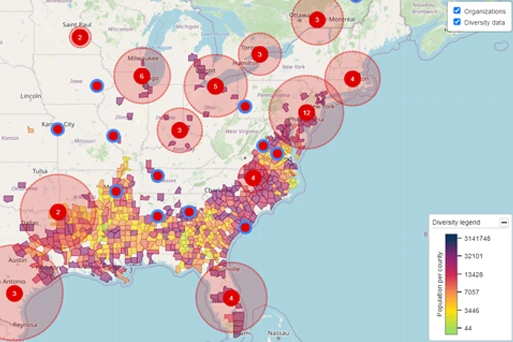
USE CASEClinical Trial Feasibility and Site Selection
TA Scan’s trial feasibility wizard and site capacity calculator support strategic decision-making during trial design, country selection, site selection, and patient allocation during the process of trial planning. With TA Scan, you save time and manual effort when identifying sites with the right capacity to successfully run your clinical trials.
Why TA Scan for Clinical Feasibility?
- Identify PIs and sites with experience
- Review the recruitment capacity of sites of interest
- Visualize diversity data alongside site experience to drive streamlined site selection and diversity strategy
- Understand competitive trial landscapes
- Generate sophisticated predictive enrollment simulations
TA Scan saves clinical teams valuable time and money, driving predictability in clinical trial timelines.

USE CASE Competitive Intelligence and Disease Landscaping
TA Scan provides an accurate, comprehensive view of global R&D so you can quickly understand and visualize therapeutic landscapes and gather ongoing competitive intelligence for your drug programs.
Why TA Scan for Competitive Intelligence?
- Quickly identify the global competitive landscape for your drugs
- Visualize ongoing competing trials and understand their study timelines
- Analyze the clinical and RWD strategies of direct competitors
- Monitor publications from competing trials, compounds, and other medical topics
TA Scan’s clinical intelligence facilitates and accelerates data-driven decision-making throughout the product lifecycle.
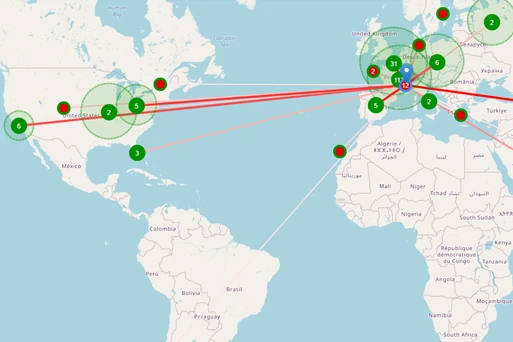
USE CASE KOL Identification and Commercial Positioning
TA Scan’s comprehensive clinical, publication, and presentation data enables commercial teams to quickly and easily understand commercial threats and supports the differentiation strategies for key compounds. Medical Affairs users can easily identify new and experienced HCPs and KOLs to support their commercial strategies.
Why TA Scan for KOL Identification and Engagement?
- Analyze, measure, and rank KOLs, speakers, and authors with TA Scan’s proprietary objective scoring
- Visualize the relevance and associations of KOLs and thought leaders
- Monitor publications and presentations from ongoing trials and competitive compounds
- Understand commercial opportunities and competitive threats
TA Scan saves teams time and manual effort, providing impactful data insights to drive successful commercial strategies
Streamline your KOL identification workflow
It's essential to engage the right thought and patient leaders to support your commercial strategy. Watch how you can quickly identify KOLs of interest with the TA Scan suite.
One Platform, Endless Data
Rely on TA Scan's robust, semantically linked database for your clinical data analysis. Get access to all the clinically relevant public data types below in one solution for maximum efficiency.
450 K+
CLINICAL TRIALS530 +
INDICATIONS2 .2M+
HEALTHCARE PROFESSIONALS330 K+
SITES10 B+
FEE DISCLOSURES7 .2M+
PUBLICATIONS730 K+
PRESENTATIONS7000 +
MODES OF ACTION260 +
DRUGS63 K+
SPONSORSClient Testimonials
"The time saved with TA Scan for a feasibility project manager is as high a 2,5 weeks research work per study project."
— Top 5 Pharma Client
"TA Scan has been pivotal to the identification and management of KOLs over the past years. The platform contains a lot of information, the navigation is very easy and intuitive, and the possibility of rating KOLs helps for early development of new drug indications."
— Top 5 Pharma Client
Do You Have a 360° Panoramic View of Your Clinical Landscape?
Make faster, data-driven decisions by searching, analyzing, and visualizing linked public and private clinical and medical data in one single source of truth to optimize your clinical trials. Get a personal TA Scan demonstration today!
Schedule A Demo




